Minerals
An expanding catalogue of minerals with descriptions of properties that will help you identify them in thin section, along with links to typical examples in Virtual Microscope samples.
This catalogue was created by Stelios Karastergios, University of Milano-Bicocca, during an internship at the Open University, with additions by Huw Sheppard, University of Oxford, during an internship at the Open University.
SHEET SILICATES (Phyllosilicates)
- BIOTITE MICA
Biotite presents a characteristic change in colour from light brown to dark brown, what geologists call a pleochroism. Usually it can show cleavage (continuous or discontinuous parallel lines) that develops parallel to the tabular appearance of biotite. Biotite is a common rock-forming mineral both in igneous but also metamorphic and sedimentary rocks.

- MUSCOVITE MICA
Muscovite mica looks like biotite under the microscope, however, there are some obvious differences that can tell which mineral is which. Muscovite is colourless (white) and occurs in assemblages that form tabular to prismatic or blade-shaped crystals. In plane polarized light, muscovite mica’s most common feature is the change of its relief when the table of the microscope is being turned around. Muscovite’s birefringence and extinction are very high and straight, respectively, just like in biotite. Muscovite is a commonly appearing mineral in all rock types (igneous, sedimentary, and metamorphic).

- CHLORITE
Chlorite is a large group of minerals that usually occur in low-grade metamorphic rocks. Chlorite is also a phyllosilicate mineral such as muscovite and biotite hence, they share some similar attributes, such as pleochroism. Chlorite’s colour is typically pale green, therefore low-grade chlorite schists have a greenish colour. Chlorite’s birefringence is peculiar because it contains colours (brownish and blueish hues) which have not been defined on the Michel-Levy scale (i.e., the scale which distinguishes the birefringence colours of the minerals). This type of birefringence is called anomalous. Chlorite can be also found in altered igneous rocks as a replacement mineral after orthopyroxene, clinopyroxene, biotite, and rarely, olivine and amphibole.

- SERPENTINE
Serpentine typically forms by alteration of other minerals like olivine, so is very often encountered as pseudomorphs of other mineral habits This can be seen in the image below, where a serpentine body follows the shape and fractures typical of the olivine crystal they replaced. It is typically colourless to dark green in plane-polarised light, with low birefringence in cross-polarised light. It can also be distinguished by very fine grains, with individual crystals combining to form larger bodies. This can create an impression of undulatory extinction. Where the alteration process is less complete, it can be found lining the edges internal cracks of olivine crystals. The effect is so pervasive that signs of alteration to serpentine can be used as an identifying feature of olivine.
Serpentine is most commonly found in mafic igneous rocks that have been exposed to water for extended periods. The upper part of the basaltic continental crust contains large amounts of serpentine, created by hydrothermal alteration of olivine. These hydrated minerals are an essential part of modern tectonics, as they transport water into subduction zones, allowing wet melting (Poli and Schmidt, 2002).
Poli, S. and Schmidt, M.W., 2002. Petrology of subducted slabs. Annual Review of Earth and Planetary Sciences, 30(1), pp.207-235.
- TALC
Talc is another phyllosilicate normally formed by alteration of other minerals. These processes include low-grade metamorphism and aqueous alteration of ultramafic rocks as well as high-temperature metamorphism of siliceous dolomites (under both high- and low-pressure conditions).
It often appears, like serpentine, as aggregates of smaller crystals filling the shape of the crystals of other minerals that it has replaced. This can be used to separate it from other white micas, like muscovite, which typically form larger, single, crystals. The key way to separate talc and serpentine is that talc is colourless in plane-polarised light. Talc also shows even higher birefringence than other white micas in cross-polarised light with colours typically of the third order.
INOSILICATES
- ORTHOPYROXENE
Orthopyroxene usually forms prismatic crystals with common cleavage. Its colour may vary from colourless to light pink or greyish depending on the rock type and included impurities (e.g., Cr, Ni and other metals). Orthopyroxene presents straight extinction and from there inherits its name, from the Greek “ortho: meaning, straight, mainly in vertical, but also horizontal position". Orthopyroxene is a common mineral component of both igneous and metamorphic rocks.
- CLINOPYROXENE
Under plane polarized light, clinopyroxene may look the same as an orthopyroxene. It appears colourless as well, and with well-defined cleavage; however, it may have a faint greenish colour. Therefore, to distinguish orthopyroxene from clinopyroxene in a rock, the usage of the crossed polarized light is needed. Compared to an orthopyroxene, a clinopyroxene crystal appears more colourful and exhibits inclined extinction. That is how clinopyroxene actually inherits its name, from the Greek “clino: meaning inclined or tilted". Clinopyroxene is also a common rock-forming mineral occurring in both igneous and metamorphic rocks.

- AMPHIBOLE
Amphibole is a large group of inosilicate minerals which contain numerous endmembers based on their mineral formulae. Under the microscope though, all amphibole endmembers share a set of common attributes. Some of these are: the formation of prismatic and rhombohedral crystals, the evident pleochroism from green to brown and the single or double cleavage (depending on the orientation of the crystal at the time of cutting). The dominant colours that amphibole has, is green and brown. Amphibole’s occurrence is very wide, including igneous and metamorphic rocks that range in a very broad pressure and temperature regime according to several geo-environments.

- GLAUCOPHANE
Glaucophane is a specific type of amphibole. It is probable the most distinctive example and is also one of the most useful for analysing the history of a unit. It is a sodic amphibole with the formula Na2(Mg3,Al2)Si8O22(OH)2. Notably, it has a pale blue colour, both in hand specimen and plane polarised light. It is so blue that rocks with large concentrations of it are called blueschists. In thin section, in addition to its distinctive blue colour, it has the definitive 60-120o cleavage found in all amphiboles, high relief and shows moderate birefringence is cross polarised light.
What makes glaucophane especially significant is its conditions of formation. It forms in metamorphic rocks which have experienced subduction, where the pressure in a rock increases rapidly but temperature only increases slowly (conditions referred to as high pressure, low temperature or HPLT). The formation of glaucophane is, in fact, regarded as the definitive diagnostic sign of subduction having occurred.
This means that blueschists are used as a primary means of identifying subduction zones. Since cold subduction is the primary unique aspect of modern terrestrial plate tectonics compared to alternative regimes, the appearance of blueschists is often used to date the onset of plate tectonics, in studies like Stern et al. (2016). However, the validity of this argument is disputed by other workers, due to variations in crustal chemistry over the 4.5Gyr history of the Earth (e.g. Palin et al. (2016)).
Stern, R.J., Leybourne, M.I. and Tsujimori, T., 2016. Kimberlites and the start of plate tectonics. Geology, 44(10), pp.799-802.
Palin, R.M., White, R.W. and Green, E.C., 2016. Partial melting of metabasic rocks and the generation of tonalitic–trondhjemitic–granodioritic (TTG) crust in the Archaean: Constraints from phase equilibrium modelling. Precambrian Research, 287, pp.73-90.
- JIMTHOMPSONITE AND CLINOJIMTHOMPSONITE
These minerals are very rare pyriboles: inoslicates formed from three conjoined chains of silicate tetrahedra. These have only been identified in a select handful of location worldwide, with one of the first being in the Far North of Scotland.
In plane polarised light, they have moderate relief and are colourless. They show a clear lozenge-shaped habit and, like amphiboles, have two non-perpendicular cleavage planes. However, unlike amphiboles, they are at 20-40/160-140o, rather than 60/120o. Birefringence is high. The key difference between the two minerals is the angle of extinction. The former has extinction parallel to the dominant cleavage while that of clinojimthompsonite is at an angle to the dominant cleavage.
NESOSILICATES
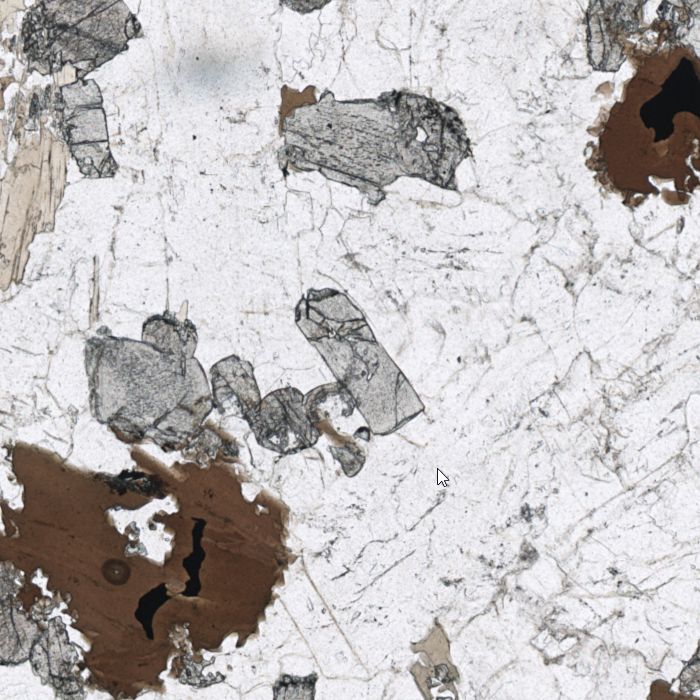
- OLIVINE
Olivine is the most common mineral in our planet since it is the main constituent of the Earth’s mantle. Macroscopically, it usually gives an olive-green colour to the rocks, hence inherits its name. Under the microscope it appears colourless, and with lots of fractures which are usually filled by secondary serpentinite (the most common olivine alteration). In crossed polars view, olivine shows colourful patterns which are characteristic of its very high birefringence. In gemmology, teardrop, or cushion-shaped cuts of olivine are called peridot and are found in many museums of natural history in the world.
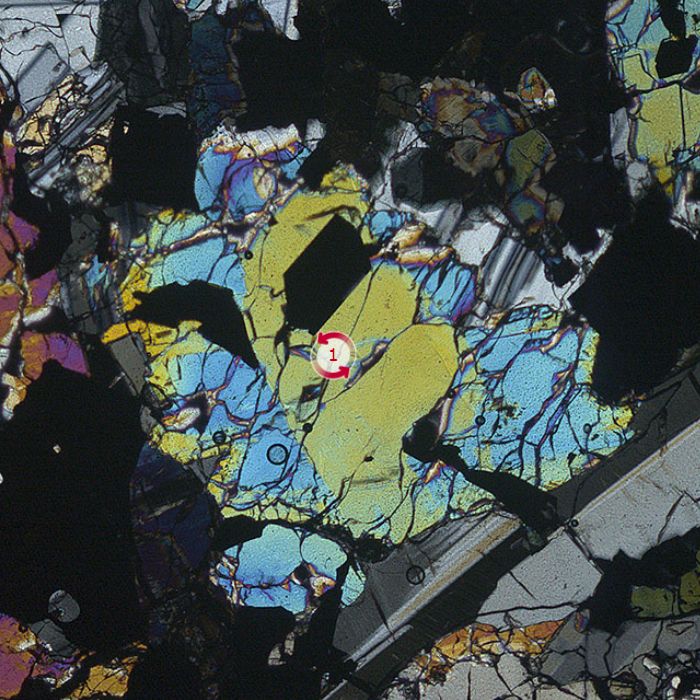
- GARNET
Garnet is a metamorphic mineral that can remain stable in a very broad range of temperatures and pressures. Magmatic garnet (i.e., crystallized from a magma) is not uncommon in igneous rocks rich in aluminium and iron. Garnet contains several endmembers based on their mineral chemistry, but the most common species are: pyrope (Mg-Al garnet), almandine (Fe-Al garnet), spessartine (Mn-Al garnet) and grossular (Ca-Al garnet). Garnet usually occurs as rounded, large grains (porphyroblasts) with high relief. Garnet belongs to the cubic crystallographic system, hence, under crossed polars shows constant extinction (i.e., opaque). Garnet is one of the most studied minerals in the world; due to its various occurrence and large stability field, it is a powerful tool for petrologists which they use very frequently in their studies. Specifically, using certain thermodynamic modelling programs they can even describe the most complex origin stories of metamorphic rocks.
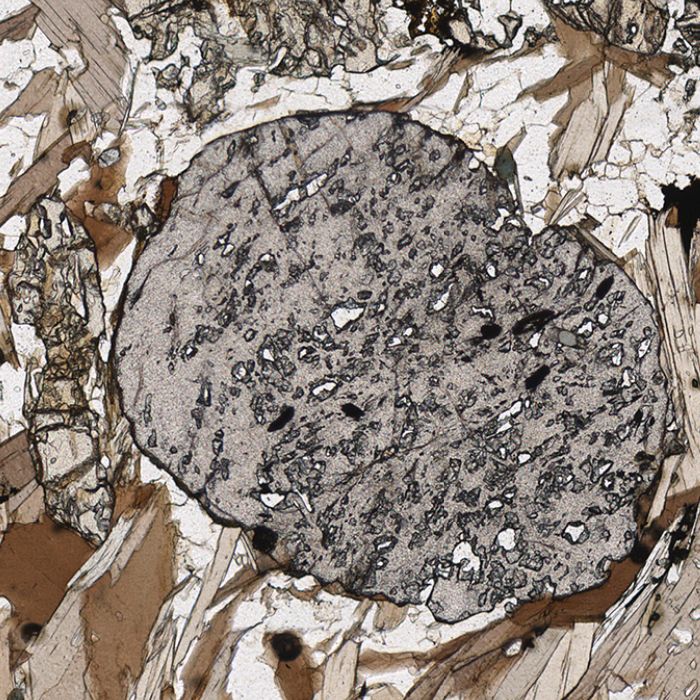
- KYANITE
Kyanite is a metamorphic mineral which is found in rocks that have been subjected and thermodynamically equilibrated under high values of pressure. In fact, geologists already know that when they find kyanite in a metamorphic rock, then this rock was buried very deep in the crust and metamorphosed. Kyanite usually forms from mineral reactions that consume minerals rich in Al (micas), during prograde metamorphism (i.e., burial in the crust). Kyanite under the microscope forms prismatic crystals with very well-defined cleavage and high relief. Another characteristic of kyanite is twinning, coupled with evident change in the birefringence for each one of the twin crystals. Kyanite, sillimanite and andalusite are all polymorphs, minerals that share the same chemical formula but formed in different conditions and/or crystallized in different crystallographic systems. Kyanite inherits its name from the Greek “kyanos”, meaning blue, which is its hand sample colour.

- ANDALUSITE
Andalusite is a metamorphic mineral which is found in metamorphic rocks that have experienced a heating episode in relatively shallow conditions (i.e., in the upper crust). Its typical microscopic appearance consists of tabular to rectangular crystals with medium relief ranging from colourless to light greyish. Of the three polymorphs (andalusite, sillimanite, kyanite), andalusite is the easiest to recognize due to a unique microscopical feature it has. The rectangular crystals frequently create an “X” pattern that crosscut each other at the centre of the crystal. This variety of andalusite is named chiastolite, as in the Greek “chiasti, meaning an X pattern”.

- SILLIMANITE
Sillimanite is a metamorphic mineral occurring in rocks that have been experienced an extreme heating episode and equilibrated under high-temperature conditions. Sillimanite is a marker mineral that geologists use to describe an event that heated up extensively the rock where sillimanite is found. Under the microscope sillimanite tends to form prismatic laths with high relief and medium cleavage. Sillimanite may look similar to kyanite; however, the birefringence of the former is much higher, presenting brighter colours. Furthermore, sillimanite may also form assemblages composed of tiny needle-like crystals. This variety of sillimanite is called fibrolite.

- ZIRCON
Zircon is an accessory mineral naturally occurring in igneous and metamorphic rocks. Based on its occurrence, in igneous rocks tends to form very small, euhedral, prismatic crystals whereas in metamorphic rocks forms rounded to ellipsoidal very small grains. Microscopically, zircon is colourless and exhibits very high birefringence colours. Zircon grains that are found as inclusions in certain minerals such as biotite and apatite, present a specific effect called pleochroic halo. In these radioactive minerals (biotite, apatite etc), zircon (which is also radioactive) develops a black halo around its extent, developing a characteristic pattern. Zircon is the most important mineral used in geochronological studies. Its ability to accommodate uranium (U) in its chemical formula (as a replacement element to zirconium), has led to the development of the uranium-lead (U-Pb) zircon geochronology method.

- TITANITE
Also known as sphene, titanite is a calcium-titanium silicate accessory mineral occurring in both igneous and metamorphic rocks. Microscopically it has a brownish to yellowish colour and forms characteristic rhombohedral-shaped crystals that look very similar to wedges. Titanite is pleochroic and has very high birefringence that sometimes "cover" the initial colour of the mineral. Although it is an accessory mineral, in titanium- and calcium-rich rocks it can form large crystals that can reach up to several centimetres in length. Titanite is an important source of titanium oxide (TiO2), a substance that has variable uses, but more importantly, that people can extract titanium out of it, which is a precious metal for modern applications in various technologies, and other general uses.

- STAUROLITE
Staurolite is a hydrated iron-aluminium silicate, found in regionally metamorphosed rocks of medium to high facies.
It is widely described as looking like cornflakes or swiss cheese. The swiss cheese effect is caused by poikiloblastic quartz. In plane-polarised light, staurolite is typically yellow and has high relief. It also shows pleochroism, turning colourless in some orientations relative to polarised light. In cross-polarised light, birefringence is low, between 1st order grey and yellow. Twinning is often observed, with crystals either at 60o or 90oto each other.
- CHLORITOID
View example in VM View example in VM
Chloritoid is a green nesosilicate mineral named for its visual similarity to chlorite. In plane-polarised light it is colourless, green, blue and shades in between, while also showing distinct pleochroism. Its relief is high and it shows low birefringence in cross-polarised light, typically shades or grey, green and blue very similar to the ppl colours.
Chloritoid is typically formed by aqueous alteration, high-pressure low-temperature (HPLT) metamorphism and low-grade regional metamorphism. Typical units are greenschist-facies, iron-and-aluminium-rich metapelites. It often occurs alongside staurolite (see second image below).
SOROSILICATES
- EPIDOTE
View example in VM (epidote)
View example in VM (clinozoisite)Epidote is a mineral that predominantly exists in metamorphic rocks; however, it might appear in igneous rocks but with a very low probability. Macroscopically, epidote has a vibrant greenish colour; however, it can also be yellowish, blueish, and even colourless. Under the microscope it presents various colours ranging from yellow to green, depending on the iron content of the mineral. Poor-iron varieties ([clino-]zoisite) may appear colourless under the microscope. The crossed polars view of epidote reveals high birefringence colours and even anomalous ones for the iron-poor endmembers.
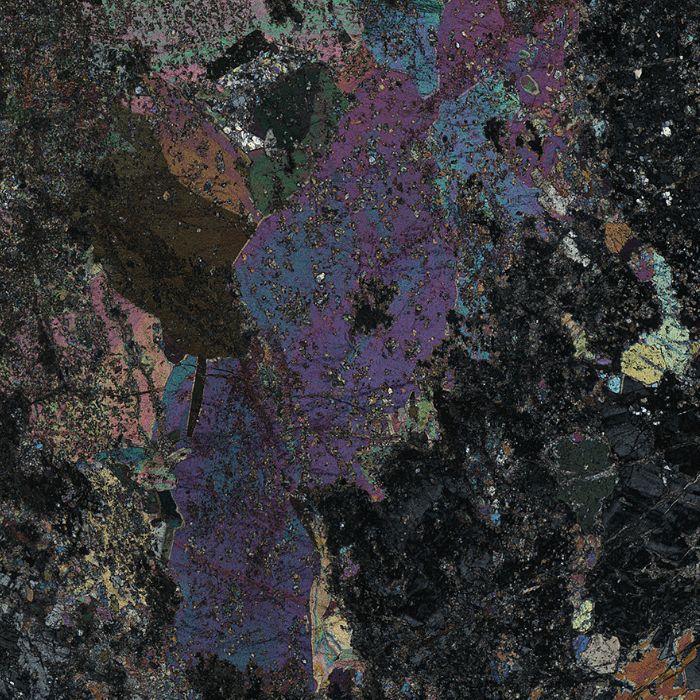
TECTOSILICATES
- QUARTZ
Quartz is the most common mineral in the Earth’s crust and appears in a plethora of rock types and different geo-environments. Quartz is a colourless mineral with low relief and low birefringence featuring greyish colours. Its extinction is never stable; in many cases, a single quartz crystal is never completely extinct in its surface. This kind of extinction is called undulose and suggests that the quartz crystals have been subjected to serious deformation and stress. Quartz is generally a very “clean” mineral, meaning that it is unaffected by any chemical alterations. However, is it common that quartz may accommodate trace element impurities which are responsible for some valuable varieties such as: tiger’s eye (yellowish hue), smoky quartz (dark grey to blackish hue) and amethyst (purple, mauve and lilac hues). Quartz is a common rock-forming mineral in all rock types, igneous, sedimentary, and metamorphic.

- ALKALI-FELDSPAR
View example in VM (Sericitization)
View example in VM (Carlsbad twinning)Alkali-feldspars (Na-K feldspars) like quartz, are very common minerals occurring in the Earth’s crust and occur in all rock types (igneous, sedimentary, and metamorphic). Under the microscope they appear as colourless minerals with low relief and low birefringence with greyish colours. They often form prismatic crystals (laths), in igneous rocks rich in alkalis and aluminium (calc-alkaline plutonic and volcanic rocks). In many rocks, alkali-feldspars are difficult to distinguish from quartz, due to their very similar relief and birefringence. Although, there are several properties that are used to separate these minerals. In contrast to quartz, alkali-feldspars are prone to alterations. The two most frequent ones are the sericitization and saussiritization. In the sericitization alteration, the alkali-feldspars are being replaced by fine-grained white mica, therefore under the microscope they obtain their birefringence and appear as large crystals with microscopic bright lights. During saussritization, alkali-feldspars are turning progressively into epidote (or epidote group minerals). Both alterations give to alkali-feldspars a “murky” kind of look. Another feature that feldspars have is twinning, where one crystal may look like a single grain in plane polarized light, but under crossed polars the feldspar is being divided into two parts. The twinning in alkali-feldspars is known as “Carlsbad twinning”.

- PLAGIOCLASE
Plagioclase is a specific feldspar group composed of a sodium-rich (Na-plagioclase) and a calcium-rich (Ca-plagioclase) endmember. Plagioclase’s occurrence is similar to quartz and alkali-feldspar, but specifically in igneous rocks, plagioclase frequently forms euhedral prismatic crystals (laths). Between the three minerals that can be found together in a rock (quartz, alkali-feldspar and plagioclase), plagioclase is the easiest to distinguish due to its very characteristic lamellae. The lamellae are visible almost always under crossed polars and appear as parallel bands with low birefringence colours ranging from light greyish to black (depending on the extinction angle). Plagioclase (like alkali-feldspar) can also be affected by sericitization and saussiritization, causing the mineral to be replaced by sericite (fine-grained white mica) or saussirite (fine-grained epidote), respectively.

CYCLOSILICATES
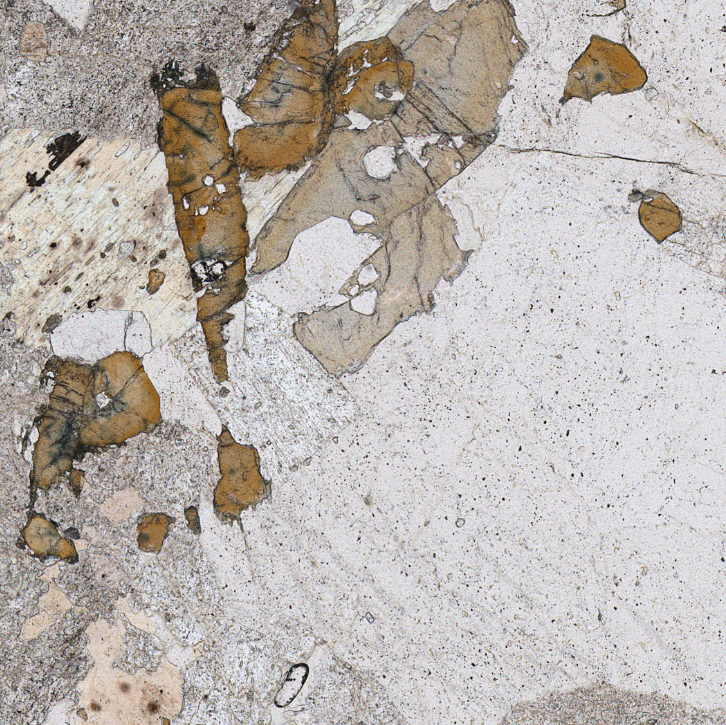
- TOURMALINE
Tourmaline is a silicate mineral notable for its high boron contents. It is primarily found in felsic igneous rocks and metamorphic rocks where boron concentrations are high. Its main identifying characteristic is that, in plane-polarised light, it shows alternating zones of pale blue and yellow. Birefringence in crossed-polarised light is moderate to high, with zoning from PPL sometimes still visible. Another frequently encountered characteristic is the rounded triangular habit found in euhedral crystals. In the perpendicular plane to this, tourmaline forms laths which create rectangles in thin section.
Where the colour alternations are unclear tourmaline can be mistaken for amphibole or pyroxene, needing careful examination of the cleavage in order to rule out these other characteristics. Differentiation may also be possible based on context: a felsic igneous rock should not contain orthopyroxene.
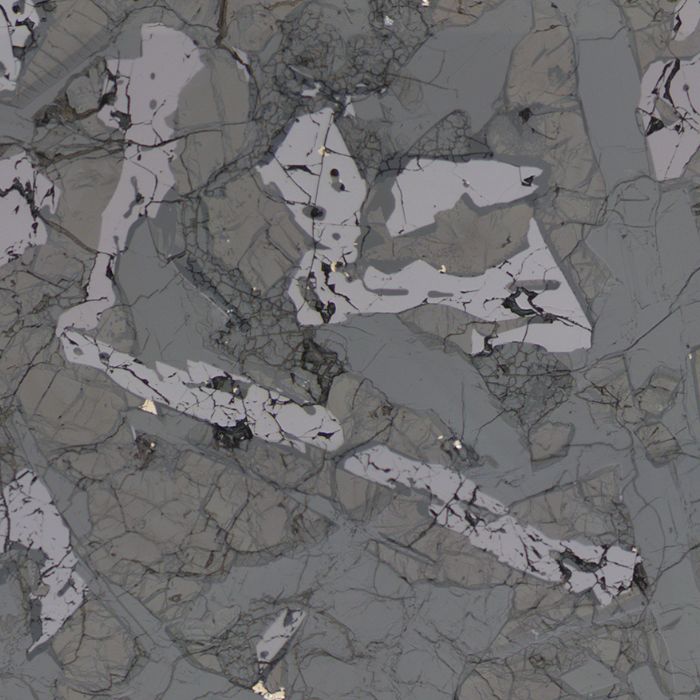
- CORDIERITE
Cordierite is cyclosilicate formed by high temperature metamorphism. It has low relief, strong pleochroism and is colourless in plane-polarised light. In cross-polarised light birefringence is low, typically black or white colours sometimes reaching first-order yellows. It often shows cyclic or lamellar twinning, both of which can be seen in the image below.
Cordierite is a definitive mineral for high-temperature (HT) metamorphism, due to either local or regional metamorphism.
CARBONATE MINERALS
- CALCITE
Calcite is a carbonate mineral, very common in sedimentary (limestones, dolostones, marls) or metamorphic rocks (marbles, skarns). It is rarely found in igneous rocks, however in igneous, ultrabasic, diamond-bearing rocks such us kimberlites and carbonatites, it is a very common rock-forming mineral. Under crossed polars is colourless and might be misunderstood for plagioclase, but the very well-defined cleavage can clear any doubt. Furthermore, it exhibits an intense change of its relief while turning the rotating table. Calcite is characterized by very high birefringence and its appearance looks like a starry night. Double cleavage is very common in calcite occurring in a rhombohedral pattern on the mineral.

- DOLOMITE
Dolomite is a calcium magnesium carbonate ((Ca,Mg)CaO3). It has a slightly different structure to calcite, with the lattice containing roughly alternating bands of Ca and Mg cations.
The origin of dolomite depends on setting and is in many cases still very ambiguous.
Under a microscope, dolomite is impossible to separate from calcite, sharing all optical and crystal characteristics. However, this can be changed by staining the slide with alizarin crimson. This is a dye which affects minerals differently based on their chemistry. Calcite is stained red/pink (best visible in plane-polarised light) while dolomite is left unstained. The image below shows the standard procedure, which is to only dip part of a slide, so that the dye doesn't make the identification of non-carbonate minerals harder. It therefore shows the difference in separating calcite and dolomite between a stained and unstained slide.
Another mineral that can be identified from staining with alizarin crimson is ferroan calcite, calcite with a high degree of iron contamination. This stains blue.
OXIDES
- HAEMATITE
Haematite is Iron (III) oxide (Fe2O3). This separates it from mganetite, which is formed from a mixture of Fe3+ and Fe2+. Haematite is chemically the same as rust, in a crystalline form.
In thin section it is typically opaque. However, when the section is cut unusually thin, it shows up with a deep red colour in both plane- and cross-polarised light. Under reflected light, it is a steel-like grey. It can adopt a variety of crystalline habits and structures including rounded botryoids.
- MAGNETITE
Magnetite is an iron oxide, and as derived from its name, it has magnetic properties. It has the chemical formula Fe3O4. This makes it unusual as it contains iron in two oxidation states, both Fe2+ and Fe3+. Magnetite crystallizes in the cubic system and therefore, develops euhedral to subhedral cubic to rounded-shaped crystals. Magnetite is a relatively heavy mineral and under the reflective light of the microscope appears with a very bright white colour.

- ILMENITE
Ilmenite is a metallic mineral composed of iron and titanium. Ilmenite is an opaque metallic mineral that crystallizes in the trigonal crystallographic system. When there is enough space and time, ilmenite forms prismatic laths or needle-like crystals. Under reflective light, ilmenite is greyish and has common cracks and fractures. Ilmenite’s reflectivity is not so bright; it is characterized by a dull greyish colour. Ilmenite is a valuable source of titanium and people have been exploited ilmenite ores for several centuries nowadays and in the past.
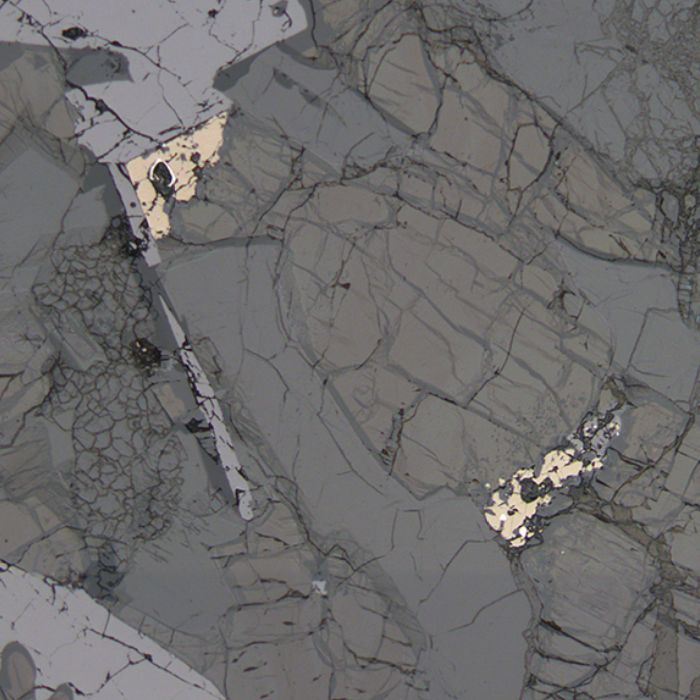
- RUTILE
Rutile is titanium oxide (TiO2). It occurs in units with high titanium concentrations, although extra titanium can be taken up by ilmenite or titanite depending on circumstances. It is primarily found in igneous and metamorphic rocks. This can be especially true in felsic rocks, as titanium favours oxide phases which are incompatible in felsic melts.
Rutile can be identified by its very dark brown colour in cross-polarised light, which also overwhelms most birefringence in cross-polarised light. However, its interference colours are, when visible, very high order.
Rutile can also form within other crystals if titanium concentrations are too high to be accommodated by the main phase. This is rutile exsolution, which mainly occurs in biotite.
Follow the link below to the 'Manual of Minerals - Features' page to see more on this effect.
- CASSITERITE
Cassiterite is tin oxide (SnO2) and provides the main source of tin for economic purposes. It is particularly notable among oxides because is not opaque to transmitted light under a petrographic microscope, so doesn't need to be studied using reflected light.
It typically shows very high relief and colours in plane-polarised light that vary between colourless, brown and red. It also shows pleochroism and very high birefringence. It also often shows internal zoning and simple twinning. The zoning can look like tourmaline but is typically much more brown rather than the blue shades found in tourmaline.
- SPINEL
Spinel is a magnesium-aluminium oxide (MgAl2O4), although it is also the name given to a group of minerals that includes magnetite and chromite (FeCr2O4).
In thin section, spinel shows high relief and dark blue (and sometimes red) colours in plane-polarised light. It is isotropic, and therefore opaque in cross-polarised light
Spinel is normally found in particularly Al-rich mafic igneous and mantle rocks. It can also be formed in high-temperature metamorphism, such as limestones that have experienced contact or regional metamorphism. Other suitable units are SiO2-poor claystones.
SULPHIDES
- PYRITE
Pyrite is a metallic mineral composed of iron and sulphur (Fe-sulphide). It is a very common accessory metallic mineral (opaque) in metamorphic and igneous rocks and crystallizes in the cubic system. Pyrite has a golden to yellowish colour under the reflective light on the microscope, which is analogous to its natural, hand-sample colour. Its microscope colour is not very bright. Due to its dull "golden" colour, it is also known as “Fool’s gold”. Pyrite ores are important iron and gold sources, as well as for production of sulphuric acid.
- CHALCOPYRITE
This is a similar mineral to pyrite, but has an additional component of copper. Its formula is CuFeS2. It is opaque to transmitted light under a microscope and has to be studied under reflected light, just like pyrite. The identifying difference between chalcopyrite and pyrite is the colour. Chalcopyrite is darker yellow than pyrite. It also shows very limited bireflectance (the reflected light equivalent of birefringence). This means identification can be difficult when only one is present.
- BORNITE
Bornite is another copper-iron sulphide (Cu5FeS4). It is opaque to transmitted light, but is dark red under plane-polarised reflected light. It appears black under cross-polarised reflected light.
PHOSPHATES
- APATITE
Apatite is a common accessory mineral in igneous and metamorphic rocks. It is one of the first minerals to form or crystallize from a magma, hence it is commonly found as mineral inclusion in larger minerals such as biotite and pyroxenes. Apatite occurs as tiny rounded or hexagonal grains with high relief. Its birefringence is similar to quartz and feldspars. A useful tip to distinguish apatite from other minerals is to look for a small mineral that looks like an ice cube, included in a larger mineral.

NON-MINERALS
- GLASS
Glass is a supercooled liquid and therefore lacks the regular, characteristic, internal structure of atoms that defines minerals. It occurs because the formation of a crystal lattice is a reaction which changes atomic bonds, meaning that it requires an activation energy. When a melt is cooled too quickly, there is not enough energy in the mixture to provide that activation energy and the crystal lattices are unable to form. Glasses are most often found in extrusive volcanic rocks, particularly basalts quenched by water (pillow basalts), where a large component of the melt is introduced suddenly to rapidly convecting fluids (water or air) which cause rapid cooling. This creates a texture of fine crystals in a matrix of dark glass.
This amorphous nature means that glass is typically translucent in plane-polarised light but opaque in cross-polarised. Colours can vary but are typically green or brown. When identifying glass it can be helpful to think about the context of the sample: in some situations glass should be expected, in others it shouldn't.
One effect that can be observed in glasses in thin section is devitrification. This is where, over time, the lattice-formation reaction has had enough energy supply to occur, but with an extremely low reaction rate. This leads to fine crystals developing in a body of glass. This can lead old basalts to consist of fine crystals in a matrix of minute crystals too small for study even under a microscope.
The sample below shows glass in a sample of fine lunar material, primarily derived from basalts and impact-derived particles (both situations which produce melts which cool rapidly). The glass on the right has adsorbed onto the surface of an olivine crystal.